MOST POPULAR
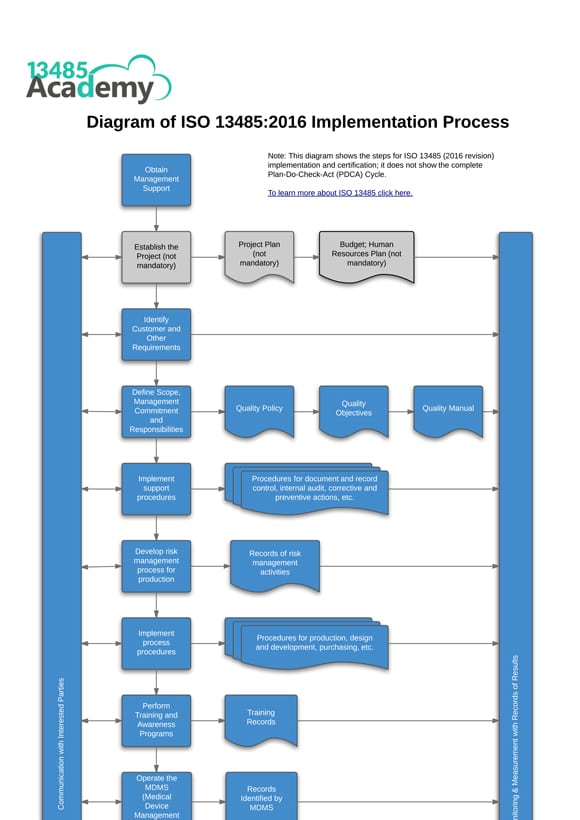
Diagram | PDF
Diagram of ISO 13485:2016 Implementation Process
Planning the implementation of ISO 13485:2016 is a crucial step in the success of your Medical Device Management System. With our ISO 13485:2016 Implementation Diagram you can see at a glance the step-by-step process to follow, ensuring nothing is forgotten.
Download

White paper | PDF
Clause-by-clause explanation of ISO 13485:2016
This document explains each clause of ISO 13485:2016 in plain English to help you better understand the requirements of the standard. The document provides guidelines on what needs to be done to meet each requirement of the standard.
Download

White paper | PDF
Checklist of Mandatory Documentation Required by ISO 13485:2016
Knowing what documents and records are necessary for ISO 13485:2016 can be confusing. This white paper is designed to clear up any misunderstandings regarding documents required by this standard, as well as outlining non-mandatory documents that are commonly used. It also offers help on structuring these documents, in a straight-forward and easy-to-follow format.
Download

Presentation | MS PowerPoint
Why ISO 13485? - Awareness presentation
This short presentation, intended for employees, shows what ISO 13485 is all about. It covers the benefits for the company, and the employees' role in different aspects of medical devices quality management.
Download
ISO 13485 White papers (12)

White paper | PDF
FDA vs. EU MDR Technical Documentation Matrix | PDF
This white paper is intended for medical device manufacturers that seek approval from the regulatory bodies. The paper describes the key differences between requirements of the U.S. Food and Drug Administration (FDA) and the MDR.
Download

White paper | PDF
8-step transition process from MDD to MDR
This white paper is intended for companies that have implemented the MDD and are planning to transition to the MDR. The paper describes the suggested steps in the transition process.
Download

White paper | PDF
EU MDR Checklist of Mandatory Documents
This white paper lists all the mandatory documentation needed by the new EU MDR regulation. The paper is intended for companies planning to sell or distribute medical devices in the European Union and want to know exactly what the regulation requires before you start.
Download

White paper | PDF
How to perform an internal audit using ISO 19011
This white paper is intended for companies that need to perform an internal audit as part of their ISO 13485 management system. Learn how ISO 19011 can help you, and read about principles of auditing, auditor characteristics, and steps for internal auditing according to this standard.
Download

White paper | PDF
How can ISO 13485 help your business grow?
This white paper is intended for quality managers, decision makers, consultants and other employees in companies planning to implement ISO 13485:2016. This helpful document gives an overview of benefits that the implementation of the ISO 13485 can bring to your company, and explains how does this standard fit with small enterprises.
Download

White paper | PDF
What to expect at the ISO certification audit: What the auditor can and cannot do
This white paper is intended for quality managers, CEOs, and consultants in companies which already implemented quality standard(s) and need guidance on what to expect at the ISO certification audit.
Download

White paper | PDF
Clause-by-clause explanation of ISO 13485:2016
This document explains each clause of ISO 13485:2016 in plain English to help you better understand the requirements of the standard. The document provides guidelines on what needs to be done to meet each requirement of the standard.
Download

White paper | PDF
How to budget an ISO 13485 implementation project
Implementing a project like ISO 13485 can be costly if you do not budget in advance. This white paper aims to help you budget effectively, and prevent any unnecessary expenses from occurring. Not only will you learn budgeting benefits and tips, but also how different implementation options can impact your overall budget.
Download

White paper | PDF
Implementing ISO 13485:2016 with a consultant vs. DIY approach
Deciding which method to follow when implementing ISO 13485:2016 can be difficult and confusing. This white paper outlines the pros and cons of both going it alone, and hiring a consultant. It offers detail on both techniques, as well as what to look for in a good online solution, helping you make an informed decision on the best approach for your business.
Download

White paper | PDF
ISO 13485:2003 vs. ISO 13485:2016 matrix
This matrix shows the relationships between the requirements of ISO 13485:2003 and the new ISO 13485:2016, while providing an overview of requirements common to both versions. It also offers tips on how to make the transition to the new version with as little stress as possible.
Download

White paper | PDF
Checklist of Mandatory Documentation Required by ISO 13485:2016
Knowing what documents and records are necessary for ISO 13485:2016 can be confusing. This white paper is designed to clear up any misunderstandings regarding documents required by this standard, as well as outlining non-mandatory documents that are commonly used. It also offers help on structuring these documents, in a straight-forward and easy-to-follow format.
Download

White paper | PDF
Twelve-step transition process from ISO 13485:2003 to the 2016 revision
This white paper is intended for companies that have implemented the ISO 13485:2003 standard, and are planning to transition to the 2016 revision. The paper describes the suggested steps in the transition process.
Download
Presentations (3)

Presentation | MS PowerPoint
Why ISO 13485? - Awareness presentation
This short presentation, intended for employees, shows what ISO 13485 is all about. It covers the benefits for the company, and the employees' role in different aspects of medical devices quality management.
Download
Presentation | MS PowerPoint
Project plan for ISO 13485 implementation presentation
Short presentation intended for quality managers, project managers, and other employees. This presentation will help clearly define the objectives of the medical devices Quality Management System implementation project, documents to be written, deadlines, and roles and responsibilities in the project.
Download
Presentation | MS PowerPoint
Project proposal for ISO 13485:2016 implementation
When it's time to present your ISO 13485:2016 implementation project to top management, you need to show them clearly and briefly why this project is important for your company. With our Project Proposal template in PowerPoint, you'll have a head start in earning management's support.
Download
Templates (2)
Template | MS Word
Project proposal for ISO 13485:2016 implementation
Implementing a project like ISO 13485:2016 is easier with the support of management. Use our project proposal template to help achieve the approval and commitment necessary from top management to progress. Plus, you'll gain invaluable insights about the project itself.
Download

Template | MS Word
Project Plan for ISO 13485 implementation
During implementation of ISO 13485, it's critical to manage all aspects of the project. You'll need to oversee everything from project milestones to individual roles and their responsibilities. With this Project Plan template, you can effectively organize your ISO 13485 implementation.
Download
Checklists (4)

Checklist | MS Word
List of questions to ask an ISO 13485 consultant List of questions to ask an ISO 13485 consultant
Before deciding which ISO 13485 expert to engage for the implementation, consider asking these questions to help you determine whether this is the right option for your company. This list contains prepared questions that are relevant to helping you find the best possible consultant for your business.
Download

Checklist | MS Word
List of questions to ask an ISO 13485 certification body List of questions to ask an ISO 13485 certification body
With so many different companies to choose from, finding the right ISO 13485 certification body to work with can be confusing. Using the prepared questions outlined in this free list, you can plan in advance exactly what to ask each prospective body. Clear explanations for each question will help you to find the perfect ISO 13485 certification body for your certification.
Download
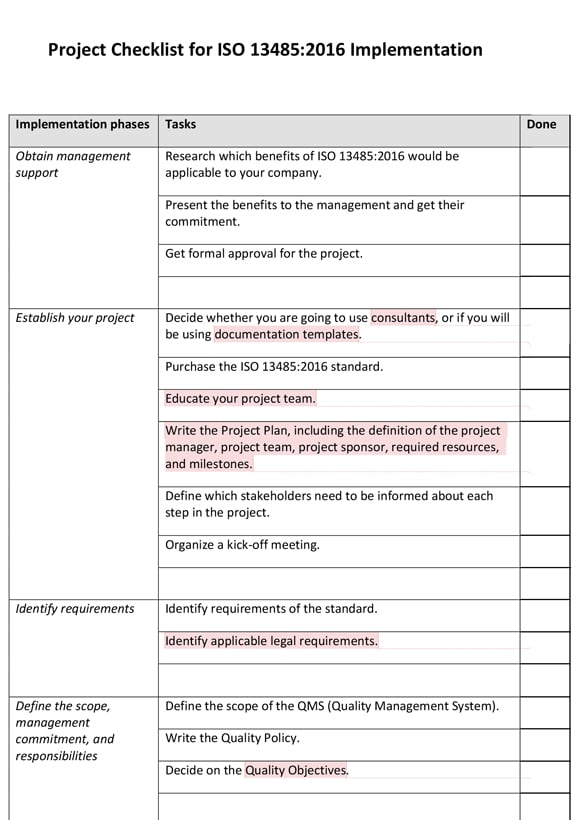
Checklist | MS Word
Project Checklist for ISO 13485:2016 Implementation Project Checklist for ISO 13485:2016 Implementation
This checklist will enable you to easily keep track of all the steps of your ISO 13485 implementation project. There are 12 major steps and 43 tasks, starting with obtaining management support all the way through to your certification audit.
Download

Diagram | PDF
Diagram of ISO 13485:2016 Implementation Process Diagram of ISO 13485:2016 Implementation Process
Planning the implementation of ISO 13485:2016 is a crucial step in the success of your Medical Device Management System. With our ISO 13485:2016 Implementation Diagram you can see at a glance the step-by-step process to follow, ensuring nothing is forgotten.
Download
Sorry, no free materials matched your criteria
Please try to search with different keywords
Source: https://advisera.com/13485academy/free-downloads/
Posted by: dianamcthigee014391.blogspot.com